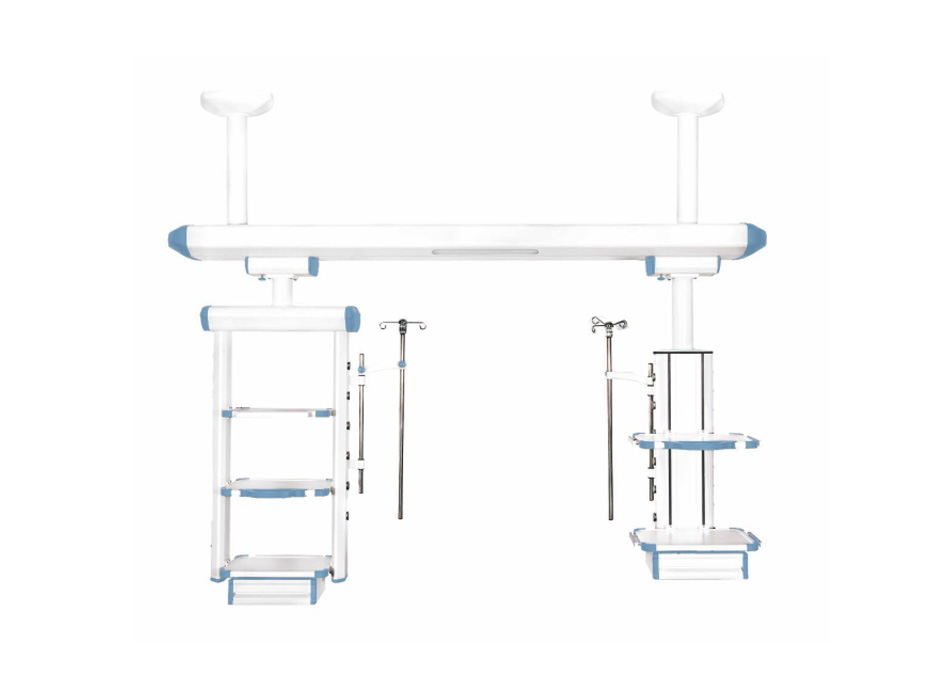
YGDQ-FY wet dry separation ICU suspension bridge
1.Working power supply: AC220V, 50Hz; The color of the suspension bridge is soft and consistent throughout, and the gaps at the junction of the profiles should be sealed. The design should be separated by gas and electricity.
2. The length of the crossbeam is 2600-3000mm (the actual size is subject to on-site measurement by the user); One lighting lamp.
3. Mechanical brake device, with no drift in the body, easy to move;
4. Suspension column type dry section tower: 1 (with a left and right movement distance of 500mm). The configuration is as follows:
(1) Instrument platform: 3 layers, the tray is formed by one-time stamping of sheet metal, and the surface is treated with anti-corrosion and rust prevention. The weight of a single tray is ≥ 50kg, 550mm * 400mm * 30mm (height adjustable); Adopting 10mm x 25mm international standard edge rail enclosure and rounded anti-collision design;
(2) Gas interface configuration (2 oxygen, 2 suction, 2 air, can be configured according to hospital requirements):
a、The interface has different colors and shapes, and has anti misconnection function;
BMore than 20000 times of plugging and unplugging; Gas terminals have scalability (optional national standards, German standards, American standards, etc.)
b、The hose on the terminal is made of PVC material, and each functional pipeline is distinguished by different colors.
(3) Power sockets: 7, 220V, 10A; Communication port: 1 RJ45 network socket, 1 telephone interface; The socket has scalability;
(4) One equipotential grounding terminal; Pipeline management tools: 2 sets;
(5) 1 stainless steel adjustable infusion rod holder; Equipped with 4 infusion bottle hooks;
(6) 1 stainless steel adjustable injection pump bracket;
(7) 1 drawer;
(8) Rotatable: 0-340 °;
(9) Dry section tower net load capacity ≥ 150kg;
(10) All gas terminals, power interfaces, and weak current interfaces are placed on the terminal box, and electricity and gas are separated,
5. Suspension column wet section tower: 1 unit (with a left and right movement distance of 500mm).
The configuration is as follows:
(1) Instrument platform: 2-layer, tray made of sheet metal stamped in one go, surface treated with anti-corrosion and rust prevention. The weight of a single tray is ≥ 50kg, 550mm * 400mm * 30mm (height adjustable); Adopting 10mm x 25mm international standard edge rail enclosure and rounded anti-collision design;
(2) Gas interface configuration (2 oxygen, 2 suction, 2 air, can be configured according to hospital requirements):
a、The interface has different colors and shapes, and has anti misconnection function;
b、More than 20000 times of plugging and unplugging; Gas terminals have scalability (optional national standards, German standards, American standards, etc.)
c、The hose on the terminal is made of PVC material, and each functional pipeline is distinguished by different colors.
(3) Power sockets: 7, 220V, 10A; Communication port: 1 RJ45 network socket with expandability
(4) One equipotential grounding terminal; Pipeline management tools: 2 sets;
(5) 1 stainless steel adjustable infusion rod holder; Equipped with 4 infusion bottle hooks;
(6) 1 stainless steel adjustable injection pump bracket;
(7) 1 drawer;
(8) Rotatable: 0-340 °;
(9) Wet section tower net load capacity ≥ 100kg;
(10) All gas terminals, power interfaces, and weak current interfaces are placed on the terminal box to fully realize the separation of electricity and gas, ensuring the safety of equipment use.
6. Each suspension tower is equipped with oxygen, negative pressure, and compressed air joints.
7. The main material is made of high-strength 6063 aluminum alloy profiles, which are molded by extrusion molding, modular splicing, and have a fully enclosed design. The surface has no sharp corners and no screws are exposed.
8. The material surface treatment adopts electrostatic spraying; Anti corrosion and easy to clean. Suitable for medical clean environments.
9. Ceiling mounted installation, stable and firm.
Product Description

Related Products
-
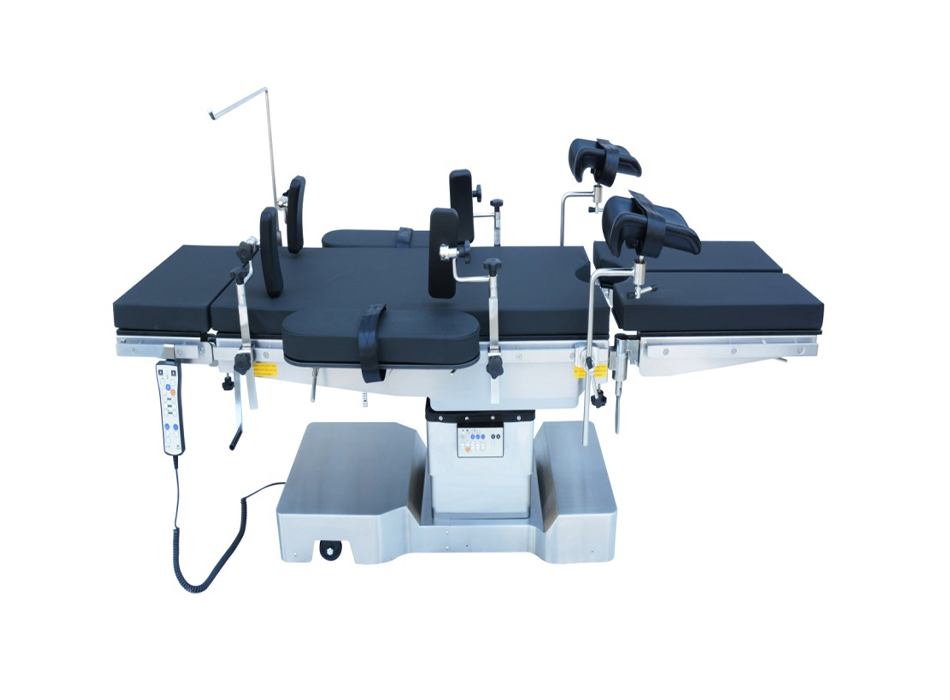
YGDH04 Ultra-low Electric hydraulic operation table
-

YGDH04A -Upgraded Electric hydraulic Operation table
-

YGDH04G Electric hydraulic surgical operating table
-

YGD02 Surgical medical electrical operating table
-

YGD03 Stainless steel electric operation table
-

YGD04 Orthopedic medical electrical operating table
Who are we?
We are a professional factory focusing on the research and development, production, sales and service of medical equipment. As a famous China YGDQ-FY wet dry separation ICU suspension bridge Manufacturers and YGDQ-FY wet dry separation ICU suspension bridge Factory, our main products include operating tables, delivery beds, hospital beds, etc., and we are committed to meeting the diverse needs of modern medical institutions.
The company is located in Rugao City, Jiangsu Province, adjacent to Shanghai, with convenient transportation. The factory covers an area of 7,600 square meters. We have an experienced R&D team that can provide OEM and customized solutions according to customer needs to meet personalized applications in different markets.
Quality is always our core. The entire production process strictly follows international industry standards to ensure that every product has reliability, durability and safety. The company has passed ISO 9001, ISO 13485, ISO 14001, ISO 45001 and other management system certifications, and all products meet CE standards.
We focus on establishing long-term cooperative relationships with customers. The professional customer service team is always available to provide you with product selection, technical support and after-sales service, truly realizing a one-stop purchasing experience, so that every customer feels at ease and trustworthy.
-
Founded In
0 -

Factory Area
0m² -
Exporting Countries
0+ -

Production Line
0line
From The Blog
Provide you with the latest enterprise and industry news
-
Electric vs. Hydraulic Operating Tables: Which is Better for Modern Surgery?
The operating room (OR) is the heart of any hospital, and the operating table is the foundation upon which every successful surgery is built. As medical technology advances, the debate between choosing a traditional hydraulic system or a modern Surgical Medical Electrical Operati...
Read More >>> 2026-02-25 -
500/700 LED vs. Single Head Lighting: Which Configuration is Right for Your Surgical Suite?
In the meticulous environment of a modern operating room, light is as essential as any surgical instrument. The ability to see fine anatomical structures with absolute clarity, without the interference of shadows or heat, is a fundamental requirement for patient safety and surgic...
Read More >>> 2026-02-18 -
Comparing LED 500 vs. Halogen Surgical Lamps: Why Mobility and LED Win in 2026
The landscape of surgical environments has undergone a quiet yet profound transformation. For decades, the warm, amber glow of halogen bulbs was the standard in operating theaters worldwide. However, as we move through 2026, a new champion has emerged: the LED 500 movable surgica...
Read More >>> 2026-02-11
 Welcome to Jiangsu Yigao Medical
Welcome to Jiangsu Yigao Medical E-MAIL:
E-MAIL:
 ENG
ENG

 English
English Español
Español



















